Photochemistry of light-responsive proteins
Light-responsive proteins have evolved to use light to drive complex processes ranging from
photosynthesis to vision. These systems not only serve as an inspiration for technology but have
also been implemented directly in biotechnologies such as bioimaging, biosensing, optogenetics,
and photodynamic therapy. However, engineering photoreceptors for use in biotechnology requires
a fundamental understanding of how they operate on a molecular level. To this end, we use hybrid
quantum mechanical/molecular mechanical models to understand how biological systems respond
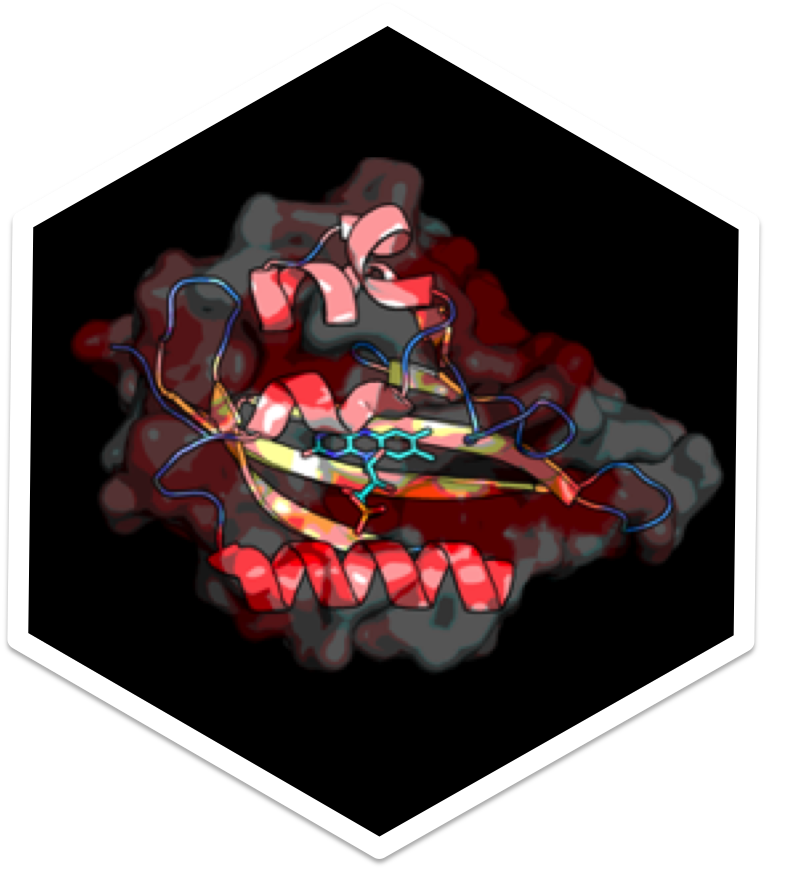
to light. We are currently focused on studying light-oxygen-voltage (LOV) domains.
Investigating new classes of fluorescent proteins
Flavin-binding fluorescent proteins (FbFPs) are recently engineered classes of
fluorescent proteins that have attractive properties. In particular, in comparison
to green fluorescent protein (GFP) derivatives, FbFPs are smaller (less genetic
content to express) and work in anaerobic conditions. We will employ hybrid
quantum mechanical /molecular mechanical (QM/MM) models to investigate
the spectral tuning mechanism and photophysics of these systems.
Modeling of photoelectron spectra
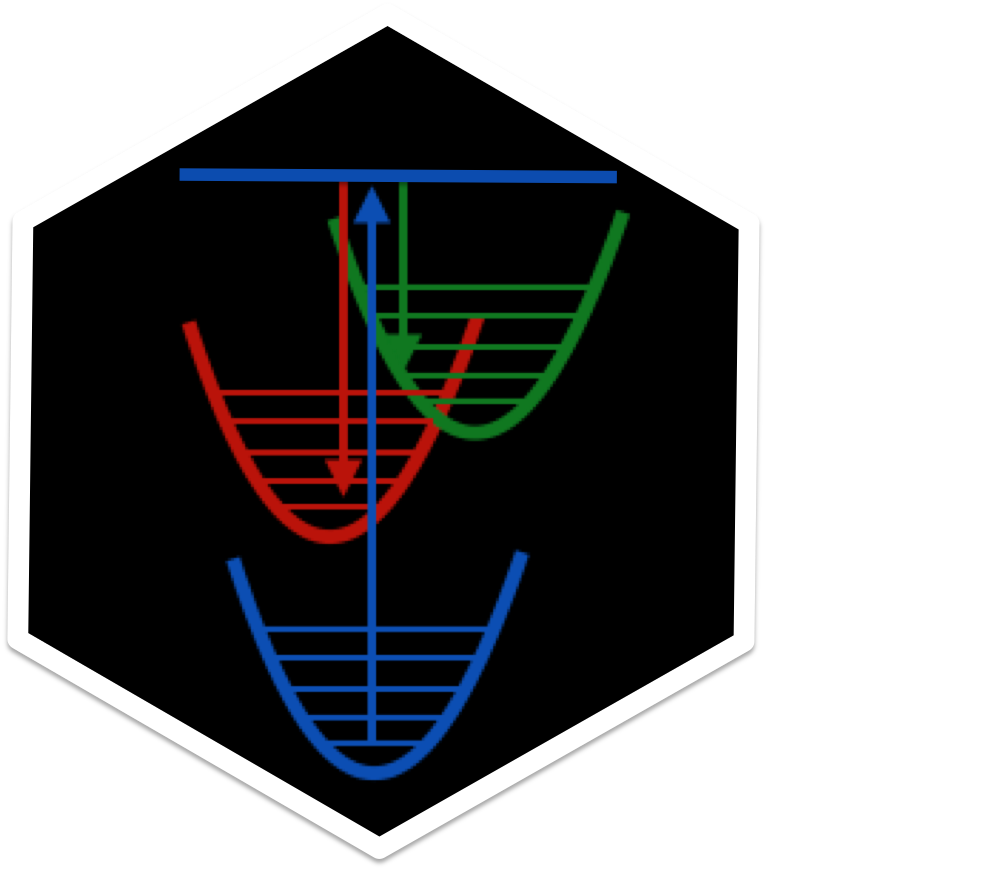
Photoelectron spectroscopy and photoelectron imaging are powerful techniques for probing the
electronic structure of molecules and ions. Time-resolved photoelectron spectroscopy, for instance,
has been employed to probe the evolving electronic character of the system through all
participating states directly. However, the interpretation of such experiments is often aided by
theoretical modeling. We are developing and applying tools for simulating and interpreting
photoelectron spectroscopy and imaging experiments.
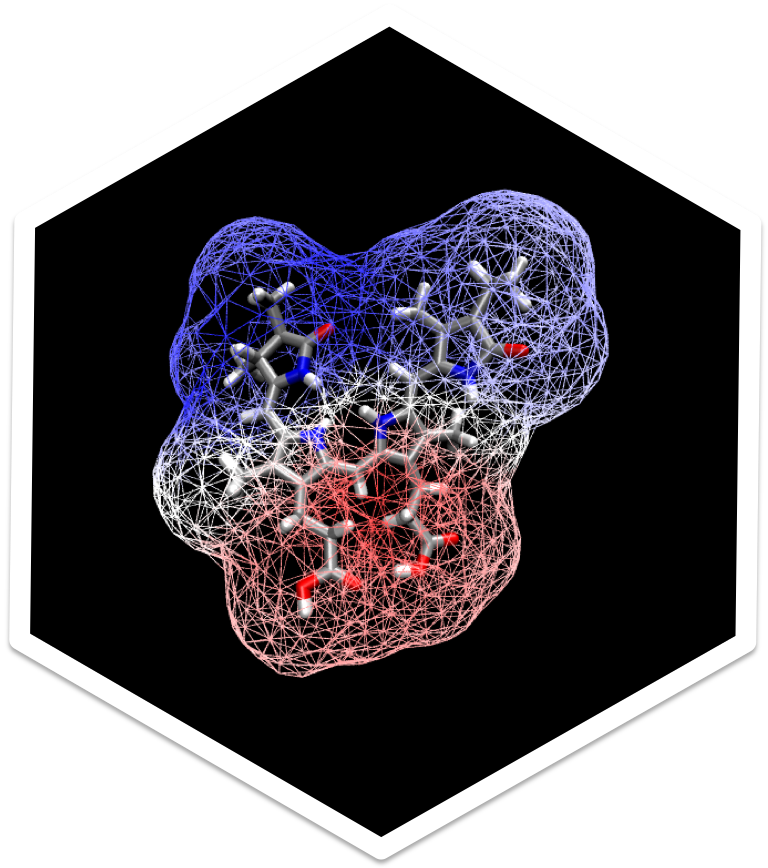
Other problems in photochemistry
We are broadly interested in modeling photophysical and photochemical
processes, and how they properties can be tuned by a solvent or
macromolecular (e.g., protein) environment.
Science Annex 504 and Natural Science Center 418
Department of Chemistry, Georgia State University, Atlanta, GA 30303.
Phone: (404)413-5569.